Tags
cooling bath, cooling baths, dry ice, freezing point depression, methanol, original research, water
Learn better with pictures? There’s a step by step writeup here.
Cooling your reaction to 0°C, -78°C or -196°C just requires ice, dry ice or liquid nitrogen. But how do you get to a temperature in between?
Mixed Solvent Systems: Freezing Point Depression
Most undergraduate labs use mixtures of ice and salt, which can range from -10 °C to -40 °C, depending on the ratio and type of salt. If you don’t have access to dry ice or liquid nitrogen this is probably the simplest option, though the two solids are irritating to mix and the setup can burn through surprisingly large amounts of salt [1].
You can also use mixtures of ethylene glycol and ethanol, which are useful from -10 °C to -70 °C (depending on the proportion of ethanol). As some of my recent research required odd temperatures, I started playing around with this system. Ultimately I found that methanol/water mixtures are far more convenient and effective.
The effective cooling range of MeOH/H2O mixtures ranges from 0 °C to -128 °C (nadir at 86% methanol). Here are a few measurements I’ve made:
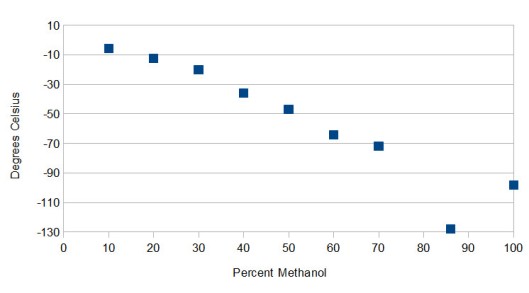
30% MeOH plateaus at -20 °C, 50% MeOH at -47 °C.
Each measurement is the warmest constant temperature, not the point of initial crystal formation. Solvent mixtures don’t actually freeze, but instead form a slurry that on cooling slowly decreases in temperature. This is because as water crystallizes out the proportion of methanol increases, leading to a new freezing point [2].
In my hands freezing ~10% of a 70:30 H2O:MeOH mixture wasn’t enough to shift the temperature of the bath more than a degree or so, but baths with higher methanol content were far less robust. At 50% methanol I found a ~5 °C wide plateau 7 °C below initial ice formation, which meant that the amount of dry ice had to be carefully controlled.
One caveat: These baths are best when brought to the desired temperature and monitored every 15 minutes or so. Adding a large excess of dry ice, as you would with an acetone/dry ice bath, will lead to a thick syrup that’s difficult to mix and far too cold. Ice still floats in the majority of methanol/water mixtures, but cubes will stay at the bottom of the Dewar if there is dry ice in their core.
The Other Options
If you need a constant, precise temperature a mixed solvent system just won’t work. If a cryocooler is out of your price range, the next best thing is to freeze a pure liquid. Water melts at 0 °C, but there are a lot of other chemicals in the lab, many of which are relatively inexpensive. Freeze one of them with dry ice, make up the volume with fresh solvent, and you have a quick and dirty cooling bath [3]. Condensation will wet the bath, so if you plan on cutting costs make sure you pour the solvent into a new bottle when you’re done, not back into the communal stock.
[1] Over the summer one of our undergraduate students went though ~7 kg of NaCl, just making -20 °C cooling baths.
[2] The exception is an 88% methanol mixture, which can be frozen solid with liquid nitrogen. Working with an 86% solution I was able to create a thick syrup of consistent temperature by rapidly stirring through freeze/thaw cycles.
[3] The wikipedia page on cooling baths is surprisingly comprehensive. Check there for specific freezing points.

Pingback: The Ridiculously Thorough Guide to Making a MeOH/Water Bath | Chemtips
Pretty helpful, thanks a lot!
Pingback: Eric’s Enlightenment for Wednesday, May 20, 2015 | The Chemical Statistician
Few interesting comments. I have foudn that using different solvents mixed with dry ice produces far different results as far as overall cooling goes. Acetone by FAR has the most cooling ability, and will blow through the most dry ice & will also evaporate much faster than say iso which will have a much lower cooling effect despite similar overall temperatures.
I use the bath in a different manner than most though, i need to use solvent in the -40c range to extract botanical material & thus must cool ~9kg of solvent through 10ft stainless coils immersed in the dry ice bath. Acetone gets the solvent down to -40 no problem while isopropyl gives nowhere near the same cooling effect. Apx-2c or so is the best i can achieve. Perhaps running the solvent through the coil slower would yield better cooling, but dealing with the acetone vapor hasnt proven to be a big enough problem
Thank you for sharing. Hope to hear more from you.
Hi Brandon,
I was wondering if you could post an update with the raw data/inputs you had for the graph shown. I’m hoping to make some cooling baths in lab, and a scale with actual values would be nice.
Sure. You can find them them on the cooling bath wikipedia page, in the table on the right-hand side of the page.
how long did it took for the undergraduate to go through the 7 kg of salt?
About 3 months, I’d say. They were only in the lab for 4.
Sorry to comment to an old blog post, but with what was the 86% methanol cooled – I am assuming it was LN2, as I don’t think Dry ice is commercially available much below -80deg C… I am after as cold as possible without LN2, Acetone or ether. There are tables saying dry ice and ether gets -100 deg C, but this is disputed by some… Not that methanol is a safe substance
“The assertion that certain baths with CO2 snow, especially the
acetone bath, give a temperature materially lower than pure dry
CO2 snow is erroneous. Cf. Theil and Caspar, Z. Physik. Chem.
86, 257-93 (1914).”
https://groups.google.com/forum/#!topic/sci.chem/D6aWRZBjls4
Hi Andre,
No trouble at all! It’s a resource, and comments are always welcome.
I used liquid nitrogen to lower the bath temp below -78 degC. To my knowledge dry ice can’t go below -78 degC, though if anything could do the job it would be diethyl ether.
Much appreciated! I am thinking if i leave a couple of knife blanks in a thickly insulated container with abundant finely divided dry ice in a deep freeze i would get the steel close enough to -78 at some point and be able to keep it there for longer… I appreciate the better heat transfer through the liquid, but what you gain on the swings…
Pingback: How can ice cool an alcoholic drink below 0°C? | Page 5 | Physics Forums - The Fusion of Science and Community
I want to make a cooling bath which can attain temperature of -55°C. The option i have is dry ice with IPA but i want to test the paint at low temperature and IPA will react with paint so i have to go with the bath that will not react with paint. The option i left with is calcium chloride bath with dry ice but it can only achieve temperature of -40°C.
Kindly suggest me appropriate cooling bath regarding above requirements.
“If you don’t have access to dry ice or liquid nitrogen” vs. “you can also use mixtures” but you need use dry ice or LN2 (e.g. “freeze one of them with dry ice”…
Any cryogenic available for -85 celcius with media ethyl alchohal